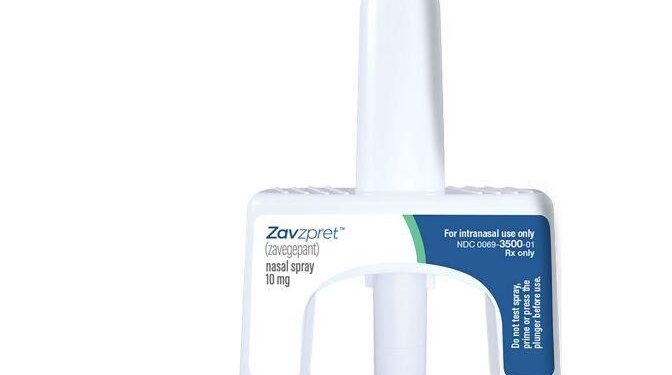
The US Food and Drug Administration has approved a new nasal spray for treatment of migraine headaches in adults.
Drug maker Pfizer in a news release said Zavzpret, also known as zavegepant, may relieve pain and other bothersome migraine symptoms as soon as 15 minutes after use.
Zavzpret works by blocking the calcitonin gene-related peptide, CGRP, a protein that is released in the brain that contributes to inflammation.
This medication is also safe for people with underlying conditions such heart disease and cannot take other migraine treatment drugs.
In one study published in the journal The Lancet Neurology, about 24% of people who took a single 10 milligram dose of Zavzpret reported they had no pain two hours later, compared with 15% of the group who got a nasal spray without any active ingredients, a difference that was statistically significant.
Also Read: Nacada Calls for Bigger Sanctions to Fight Illicit Brew
Side effects, which is a 1 in 5 cases, is an altered sense of taste.
“When a migraine hits, it has a significant negative impact on a person’s daily life,”
“Among my migraine patients, one of the most important attributes of an acute treatment option is how quickly it works. As a nasal spray with rapid drug absorption, Zavzpret offers an alternative treatment option for people who need pain relief or cannot take oral medications due to nausea or vomiting, so they can get back to normal function quickly.” Dr. Kathleen Mullin, the associate medical director at New England Institute for Neurology & Headache who has studied the drug, said in the news release from Pfizer.
The drug is expected to be available in pharmacies in July 2023, the company said