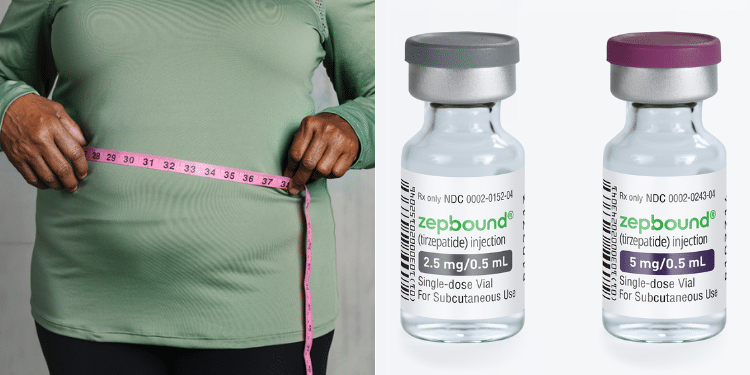In November 2023, the U.S Food and Drug Administration (FDA) approved Zepbound (tirzepatide), an injectable prescription medicine that may help adults with obesity, excess weight (overweight) or those who have weight-related medical problems, lose weight and keep it off.
The drug is intended for adults with a body mass index (BMI) of 30 kilograms per square meter (kg/m²) or greater for obesity, or a BMI of 27 kg/m² or greater for overweight, provided they also have at least one weight-related condition, such as high blood pressure, type 2 diabetes, or high cholesterol.
It should be used with a reduced-calorie diet and increased physical activity.
Zepbound activates receptors of hormones secreted from the intestine (glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)) to reduce appetite and food intake.

Dosage of Zepbound Drug and How its Administered
The weight loss drug is administered via injection under the skin once a week, with a recommended starting dosage of 2.5 mg.
After 4 weeks, the dosage can be increased to 5 mg injected subcutaneously once a week. Further dosage increments of 2.5 mg can be made after at least 4 weeks on the current dose.
The recommended maintenance dosages are 5 mg, 10 mg, or 15 mg, all administered subcutaneously once a week. The maximum dosage for Zepbound is 15 mg once weekly.
A person can administer once weekly at any time of day, with or without meals and inject subcutaneously in the abdomen, thigh, or upper arm. Rotate injection sites with each dose.
Clinical trial data found that people using 5-mg injections of tirzepatide lost about 15% of their weight after more than a year of treatment, on average.
Also Read: Kenyans Warned Against Buying Popular Ulcer Drug
Zepbound Manufacturer Reduces Drug Prize
On August 27, Eli Lilly and Company, the manufacturer of Zepbound, announced the availability of 2.5 mg and 5 mg single-dose vials for self-pay patients with an on-label prescription.
These vials are priced at a 50% or greater discount compared to the list price of other incretin (GLP-1) medications for obesity.
This new option aims to help millions of adults with obesity access the medication, particularly those not eligible for the Zepbound savings card program, those without employer coverage, and those who need to self-pay outside of insurance.
A four-week supply of the 2.5 mg Zepbound single-dose vial is priced at $399 (approximately Ksh. 51,000), while the 5 mg dose is available for $549 (approximately Ksh. 70,400).
These prices are less than half the list price of other incretin medications for obesity and align with the Zepbound savings program for individuals not covered by insurance.
What You Should Know Before using the Weight Loss Drug
Your healthcare provider should show you how to use Zepbound before you use it for the first time.
Tell your doctor if you are taking medicines to treat diabetes including insulin or sulfonylureas which could increase your risk of low blood sugar.
If you take birth control pills by mouth, talk to your healthcare provider before you use Zepbound. Birth control pills may not work as well while using the drug.
Your doctor may recommend another type of birth control for 4 weeks after you start Zepbound and for 4 weeks after each increase in your dose of Zepbound.
Also Read: Interpol Sends Warning to Govt Against Fake Diabetes Drugs
Side Effects of the Drug
Severe Stomach Problems: Users may experience severe stomach issues. Contact your healthcare provider if you have severe or persistent stomach problems.
Kidney Problems: Diarrhea, nausea, and vomiting can lead to dehydration, potentially causing kidney issues. It’s crucial to stay hydrated to reduce this risk.
Gallbladder Problems: Some users have reported gallbladder issues. Seek immediate medical attention if you experience upper stomach pain, fever, jaundice (yellowing of skin or eyes), or clay-colored stools.
Pancreatitis: If you experience severe abdominal pain that doesn’t go away, with or without vomiting, stop using Zepbound and contact your healthcare provider.
Serious Allergic Reactions: Seek emergency medical help for symptoms of a serious allergic reaction, including swelling of the face or throat, difficulty breathing, severe rash, or rapid heartbeat.
Low Blood Sugar (Hypoglycemia): Using Zepbound with medications that lower blood sugar may increase your risk of hypoglycemia. Symptoms include dizziness, sweating, confusion, headache, and rapid heartbeat.
Vision Changes: Inform your healthcare provider of any changes in vision while using Zepbound, especially if you have type 2 diabetes.
Depression or thoughts of suicide. You should pay attention to changes in your mood, behaviors, feelings or thoughts and contact a doctor.
Other most common side effects of Zepbound include nausea, diarrhea, vomiting, constipation, stomach (abdominal) pain, indigestion, injection site reactions, feeling tired, allergic reactions, belching, hair loss, and heartburn.
Zepbound may cause tumors in the thyroid, including thyroid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, tell your healthcare provider.
Who is not Allowed to Use
Do not use Zepbound if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) and if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Zepbound if you have had a serious allergic reaction to tirzepatide or any of the ingredients in the drug.
FDA approved the drug only for use in people 18 years of age and older.
Follow our WhatsApp Channel for real-time news updates!
https://whatsapp.com/channel/0029VaB3k54HltYFiQ1f2i2C









![Billionaire Richard Branson Invites Guests For Ksh256,000 Hike, Breakfast In Kenya [Full Package] Luxury Getaway Establishment By Finch Hattons Photo/Finch Hattons](https://thekenyatimescdn-ese7d3e7ghdnbfa9.z01.azurefd.net/prodimages/uploads/2024/05/Tsavo-3.jpg)