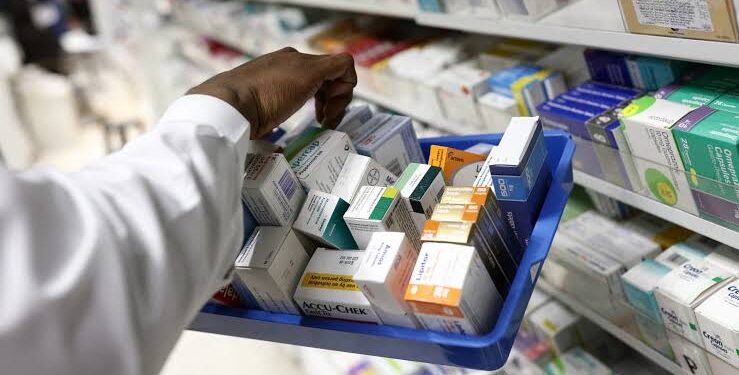
The Pharmacy and Poisons Board (PPB) has ordered the quarantine of a popular cancer drug named Flurasted 500 (5-Fluorouracil) Injection Batch no. HHP24017.
PPB in a statement on Thursday, December 5, stated that the quarantine order is being issued due to a market complaint on the appearance parameter of the content.
The board directed all pharmaceutical outlets, healthcare facilities, professionals, and members of the public to immediately stop the further distribution, sale, issuance, or use of the affected product batch.
“The Pharmacy and Poisons Board (“the Board”) orders the quarantine of Flurasted 500 (5-Fluorouracil) Injection Batch no. HHP24017 Manufactured by Halsted Pharma Private Limited, India,” PPB said.
“In light of this, the Board advises all pharmaceutical outlets, healthcare facilities, healthcare professionals, and members of the public to immediately quarantine the product batch and STOP the further distribution, sale, issuance, or use of the affected batch.”

PPB Orders Quarantine of Popular Cancer Drug Flurasted
Flurasted 500 (5-Fluorouracil) Injection is used in the management of cancer.
The Board encouraged the public to report any suspected cases of sub-standard medicines or adverse drug reactions to the nearest healthcare facility or the Pharmacy and Poisons Board.
Also Read: Govt Recalls Drug Over Labelling Mix-Ups
This can be done through the following channels: Online https://pv.pharmacyboardkenya.org/users/mpublic, USSD code at *271#, Mobile application: mPvERS both Android & iOS Email [email protected] or [email protected] of telephone No. 0795743049.
“The Board is committed to ensuring the safety and efficacy of medicines in the market to protect public health,” PPB added.
Earlier Recalls Over Labeling Errors
The recent quarantine order from the Pharmaceuticals and Poisons Board (PPB) follows a recall order issued barely a week earlier for two batches of Efinox Nasal Drops (Batch No. 82979 for the 1% strength and Batch No. 82978 for the 0.5% strength).
PPB said the recall was prompted by a labeling mix-up where the correct product was identified, but the wrong strength was applied to the packaging.
Laboratory and Allied Ltd, the manufacturer, conducted an investigation and concluded that a mix-up occurred between the 0.5% and 1% w/v strengths during the labeling and packing process for these specific batches.
Also Read: Zepbound: What You Need to Know About the Weight Loss Drug and How It Works
In September, the Pharmaceuticals and Poisons Board (PPB) issued a recall for Flamodip tablets, manufactured by Medico Remedies, due to a labeling error.
The mistake was that the secondary packaging of the product was labeled as “Flamodip-5 (Amlodipine),” while the primary packaging was incorrectly labeled as “Flamodip-5 (Enalapril).”
Flamodip-5 (Amlodipine) is commonly prescribed to treat high blood pressure in adults and children aged six years and older.
On the other hand, Enalapril is used to treat high blood pressure and heart failure (the weakening of heart function).
Follow our WhatsApp Channel and join our WhatsApp Group for real-time news updates.